Global Guidelines and Pipeline Updates
Week Of November 29 – December 5, 2025
This was a relatively slow week for new announcements, which gave me space to focus on policy and early-stage science. I’ve added a note on global health policy to highlight the WHO’s latest guidance and the potential access gap for GLP-1s. I am also including a new section this week on pipeline updates. While Phase 3 trials often get the majority of attention, I wanted to highlight a few Phase 2 and Phase 4 trials -- including one looking at chemotherapy-related nausea -- that show the expanding range of indications being tested.
🌍 GLOBAL HEALTH POLICY
WHO issues first global guideline recommending GLP-1s for obesity treatment — The World Health Organization formally recognized obesity as a treatable chronic disease and issued its first guidance endorsing GLP-1 therapies (semaglutide, liraglutide, tirzepatide) for long-term weight management. The recommendation is conditional, citing limited long-term safety data and calling for combination with behavioral interventions. WHO highlighted a stark access gap: even with expanded production, fewer than 10% of those who could benefit will have access by 2030.
[Policy Milestone]
🆕 NEWLY REGISTERED TRIALS (1 in last week)
Tirzepatide Phase IV trial investigating its role in postmenopausal HR+ breast cancer survivors (Weill Medical College of Cornell University, n=30)
[Weight Loss/Efficacy | New Indications]
Trials: NCT07257484 | Mechanism: Dual GLP-1/GIP agonist
📋 PIPELINE UPDATES
Novo Nordisk amycretin Phase 2 completes — Dose-finding study (n=448) for NNC0487-0111 in type 2 diabetes finished enrollment. Next-gen GLP-1/amylin dual agonist showed 22% weight loss in earlier obesity trials.
[Completed] NCT06542874
Semaglutide depression trial now recruiting — Danish study exploring whether semaglutide improves depressive symptoms in patients with MDD and overweight/obesity (n=116).
[Now Recruiting] NCT07136714
Lilly’s LY3537021 begins enrollment — Phase 2 trial for chemotherapy-induced nausea/vomiting now recruiting, representing potential expansion of GLP-1 mechanisms into supportive oncology care.
[Now Recruiting] NCT07169851
💡 TRIAL SPOTLIGHT
Educational spotlight selected by editors
Amycretin (NNC0487-0111) in Type 2 Diabetes
This Phase 2 dose-finding study evaluated “amycretin” (NNC0487-0111), a single molecule engineered to activate both GLP-1 and amylin receptors simultaneously. Unlike combination therapies that mix two separate drugs, amycretin is a unimolecular co-agonist designed to leverage the complementary effects of GLP-1 (insulin secretion, appetite suppression) and amylin (satiety, gastric emptying regulation) for potent glycemic control and weight management. Enrolling 448 participants with Type 2 diabetes, the trial investigated whether this dual-mechanism approach could outperform standard care in lowering HbA1c and body weight. The successful completion of this study is strategically critical for Novo Nordisk, as it sets the stage for Phase 3 development of a potential “next-generation” successor to semaglutide. By targeting the amylin pathway alongside GLP-1, this asset aims to break efficacy ceilings seen with mono-agonists, offering a more comprehensive metabolic intervention for patients requiring significant weight loss alongside glucose regulation.
🔬 MECHANISM EXPLAINED
Understanding the science behind the therapeutics
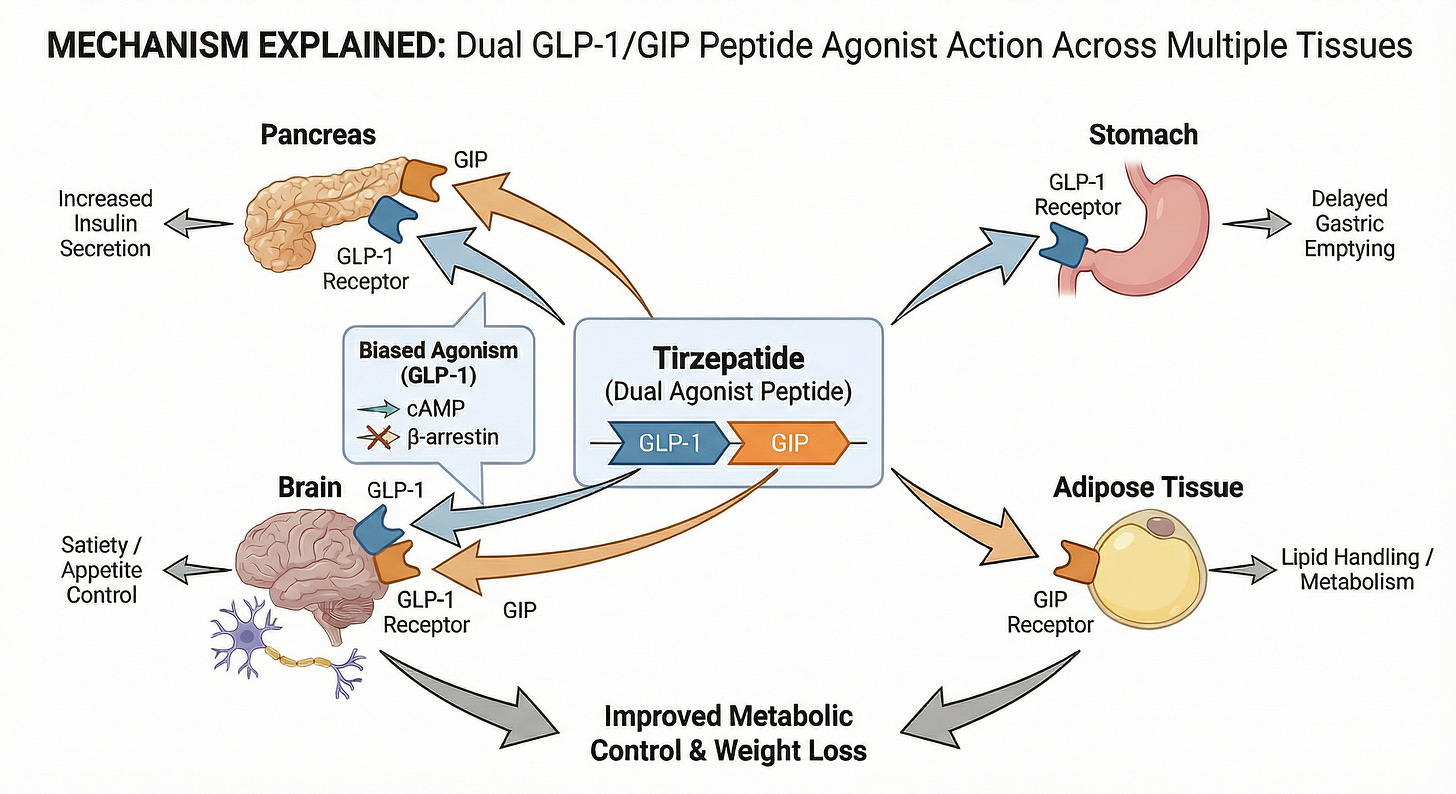
Dual GLP-1/GIP agonist (Example drug: Tirzepatide)
Tirzepatide represents a pivotal evolution in incretin therapy as a unimolecular dual agonist that simultaneously engages both glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors. While the GLP-1 component drives satiety and glucose-dependent insulin secretion, the addition of GIP signaling works synergistically to amplify metabolic regulation and lipid handling, potentially through direct modulation of adipose tissue. Uniquely, tirzepatide exhibits biased agonism at the GLP-1 receptor—favoring cAMP generation over β-arrestin recruitment—which may enhance tolerability while maximizing the therapeutic window compared to selective mono-agonists. This “twincretin” strategy not only resets the efficacy benchmark for weight loss and glycemic control but also validates the broader industry shift toward multi-receptor modalities as the future standard of care in metabolic disease. (The diagram below omits indirect effects, for example those on the liver and on skeletal muscle).
This newsletter compiles publicly available information from company press releases and ClinicalTrials.gov. Not investment advice.