CagriSema and Orforglipron Head to FDA
Week Of December 13 – December 19, 2025
This week, we had two major FDA submissions within days of each other. Novo Nordisk filed CagriSema for weight management, and Lilly submitted orforglipron. For the latter, positive ATTAIN-MAINTAIN data showed the oral GLP-1 can help patients maintain weight loss when switching from injectables. That seems to me like a pretty big deal, given that a significant fraction (figures vary but as high as 50%) of those taking GLP-1s stop them within a year and often regain much of the weight they lost (pubmed). Add in Altimmune’s pemvidutide advancing toward Phase 3 for MASH and it was a busy week for the pipeline.
For the Trial Spotlight, I chose Lilly’s eloralintide ENLIGHTEN-2 study -- a selective amylin agonist that represents Lilly’s bet on a GLP-1-sparing approach. The Mechanism Explained section covers the scientific rationale of combining amylin with GIP activation, a pairing that could offer an alternative for patients. The reality is that people respond differently to different weight loss drugs and so having alternatives that are potentially better tolerated may be important.
🔥 THIS WEEK’S KEY DEVELOPMENTS
Novo Nordisk submits CagriSema to FDA for weight management approval.
Novo Nordisk has submitted a New Drug Application (NDA) to the U.S. FDA for once-weekly CagriSema (cagrilintide 2.4 mg and semaglutide 2.4 mg) for long-term weight management in adults with obesity or overweight. The submission is based on the REDEFINE clinical program, including the REDEFINE 1 phase 3 trial, where CagriSema demonstrated an average body weight loss of 23%. The FDA is expected to review the application in 2026.
Press | Trials: NCT05394519 | NCT05567796 | NCT05669755 | Mechanism: GLP‑1 and amylin combination
Lilly submits oral weight loss drug orforglipron to FDA.
In the Phase 3 ATTAIN-MAINTAIN trial, Lilly’s once-daily oral orforglipron met its primary and all key secondary endpoints for weight maintenance versus placebo at 52 weeks. The study evaluated participants who switched to orforglipron after achieving weight loss on injectable Wegovy or Zepbound. At 52 weeks, participants switching from Wegovy maintained all but 0.9 kg of their previously achieved weight loss on average, while those switching from Zepbound had an average difference of 5.0 kg. Lilly has submitted orforglipron to the U.S. FDA for the treatment of obesity.
Press | NCT06584916 | Mechanism: oral GLP-1 receptor agonist
Positive MASH data advances Altimmune’s pemvidutide to Phase 3.
Altimmune announced positive 48-week topline results from its IMPACT Phase 2b trial of pemvidutide in patients with MASH. The 1.8 mg dose achieved a mean reduction in Liver Stiffness Measurement (LSM) of -3.97 (p<0.001) and a mean weight loss of 7.5% (p<0.0001), compared to -0.03 and 0.2% for placebo, respectively. The discontinuation rate due to adverse events was 1.2% for the 1.8 mg dose versus 3.5% for placebo, and the company plans to initiate a Phase 3 program in 2026.
Press | Trials: NCT05989711 | Mechanism: glucagon/GLP-1 dual receptor agonist
📰 PRESS RELEASES
Structure Therapeutics begins Phase 1 trial for oral obesity drug ACCG-2671.
Press | Mechanism: amylin receptor agonist
🆕 NEWLY REGISTERED TRIALS (16 in last week)
Eloralintide Phase 3 investigating its use in adults with obesity or overweight and type 2 diabetes (Eli Lilly, n=1035)
[Weight Loss/Efficacy | Oral Formulations]
Trials: NCT07282600 (ENLIGHTEN-2) | Mechanism: Amylin receptor agonist
KAI-7535 Phase 1 investigating the effect of a low-fat meal and evening dosing in participants with obesity or overweight (Kailera, n=18)
[Weight Loss/Efficacy]
Trials: NCT07294898 | Mechanism: GLP-1 receptor agonist
GZR18 Injection Phase 3 extension study evaluating long-term safety and efficacy for weight loss in participants from a prior trial (Gan & Lee Pharmaceuticals, n=500)
[Weight Loss/Efficacy]
Trials: NCT07292441 (GZR18-BWM-303) Mechanism: GLP-1 receptor agonist
Tirzepatide Phase 2 trial investigating the drug plus cognitive-behavioural therapy (CBT) for Alcohol Use Disorder in adults with obesity (South West Sydney Local Health District, n=46)
[Weight Loss/Efficacy]
Trials: NCT07292519 | Mechanism: Dual GLP-1/GIP agonist
GALACTUS Trial Phase 3 investigating the effect of GLP-1 and SGLT2 inhibitors on inflammation following myocardial infarction (Instituto Mexicano del Seguro Social, n=44)
[New Indications]
Trials: NCT07295223 (GALACTUS) | Mechanism: GLP-1 RA as comparison
Semaglutide and Tirzepatide trial investigating their effect on genetic aging delay in adults with obesity (Second Affiliated Hospital, Zhejiang University, n=66)
[Weight Loss/Efficacy]
Trials: NCT07293325 | Mechanism: GLP-1 / GIP dual and GLP-1 receptor agonist
Semaglutide Phase 3 studying the cardiometabolic and physiological effects after patients stop taking the drug (Mount Sinai Hospital, Canada, n=98)
[Weight Loss/Efficacy | New Indications]
Trials: NCT07294950 (REST) | Mechanism: GLP-1 receptor agonist cessation
KAI-9531 Phase 3 trial investigating a new drug for weight management in adults with obesity or overweight who do not have diabetes (Kailera, n=1800)
[Weight Loss/Efficacy | Safety/Tolerability]
Trials: NCT07284875 | Mechanism: Dual GLP-1/GIP agonist
KAI-9531 Phase 3 trial evaluating the once-weekly drug in participants with both obesity/overweight and diabetes (Kailera, n=1700)
[Weight Loss/Efficacy | Safety/Tolerability]
Trials: NCT07284901 | Mechanism: Dual GLP-1/GIP agonist
KAI-9531 Phase 3 trial investigating the once-weekly drug against semaglutide and placebo for adults with obesity without diabetes (Kailera, n=1200)
[Weight Loss/Efficacy | Safety/Tolerability]
Trials: NCT07284979 | Mechanism: Dual GLP-1/GIP agonist
Tirzepatide Phase 2 trial investigating its use to improve automated glycemic control in adults with Type 1 Diabetes (Melissa-Rosina Pasqua, n=105)
[New Indications]
Trials: NCT07284511 (TZP) | Mechanism: Dual GLP-1/GIP agonist
Ecnoglutide (VRB-101) Phase 2 trial evaluating a weekly oral formulation for adults with obesity or overweight (Verdiva Bio Dev Limited, n=200)
[Weight Loss/Efficacy | Oral Formulations]
Trials: NCT07281937 (EVOLVE-2) | Mechanism: GLP-1 agonist
Semaglutide (Wegovy) Phase 2 pilot study investigating the popular GLP-1 agonist for trichotillomania, an impulse control disorder (University of Chicago, n=10)
[New Indications]
Trials: NCT07282769 | Mechanism: GLP-1 receptor agonist
HRS9531 Phase 3 head-to-head study comparing efficacy and safety against semaglutide for obesity (Fujian Shengdi Pharmaceutical, n=750)
[Weight Loss/Efficacy | Safety/Tolerability]
Trials: NCT07285902 | Mechanism: Dual GLP-1/GIP agonist
CagriSema Phase 3 trial investigating its effect on blood sugar and body weight in children and adolescents with Type 2 Diabetes (Novo Nordisk A/S, n=80)
[Weight Loss/Efficacy]
Trials: NCT07282613 (REIMAGINEYOUNG) | Mechanism: GLP-1/amylin combo
Dulaglutide Phase 2 trial investigating the GLP-1 RA’s effect on severe intracranial atherosclerosis (National University of Singapore, n=130)
[New Indications]
Trials: NCT07282041 (RADIANT) | Mechanism: GLP-1 receptor agonist
💡 TRIAL SPOTLIGHT
Educational spotlight selected by editors
A Study of Eloralintide (LY3841136) in Participants With Obesity or Overweight, and Type 2 Diabetes (ENLIGHTEN-2)
This Phase 3 trial (NCT07282600), known as ENLIGHTEN-2, evaluates Eli Lilly’s eloralintide in 1,035 participants with type 2 diabetes and obesity or overweight. Eloralintide operates via a novel mechanism distinct from GLP-1 agonists; it is a selective amylin receptor agonist (preferentially targeting AMY1R) designed to induce satiety and weight loss while potentially minimizing the gastrointestinal side effects associated with non-selective amylin analogs. The ~75-week, randomized, placebo-controlled study will assess the drug’s efficacy in weight reduction, a crucial endpoint given that weight loss is often harder to achieve in patients with type 2 diabetes compared to those without. Strategically, this trial positions Lilly to compete directly with other emerging amylin-based therapies (such as Novo Nordisk’s cagrilintide) and diversifies the company’s obesity portfolio beyond incretin-based treatments like Zepbound (tirzepatide), offering a potential alternative for patients who may not tolerate or respond adequately to GLP-1 therapies.
🔬 MECHANISM EXPLAINED
Understanding the science behind the therapeutics
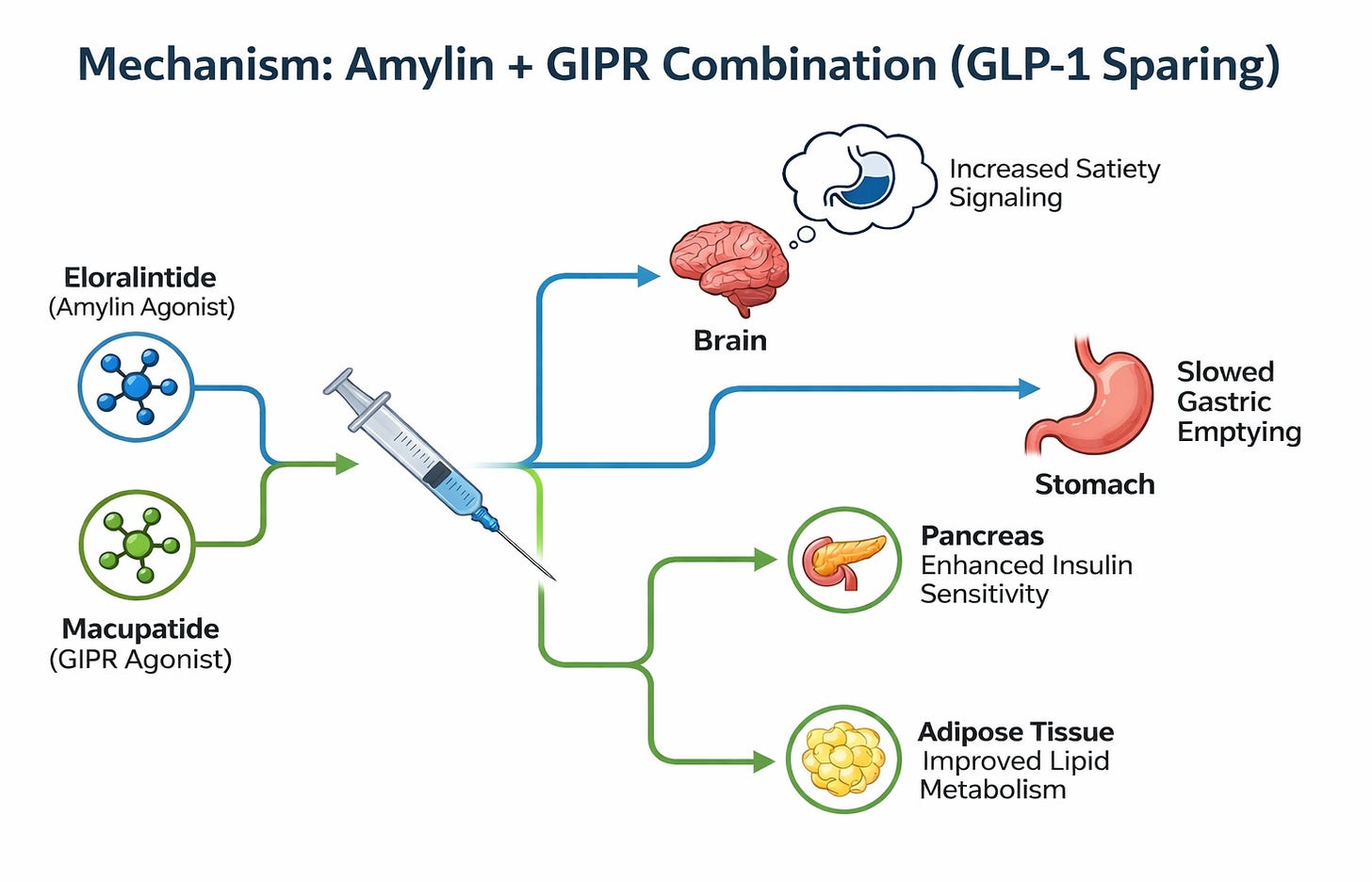
Amylin + GIPR Combination (Example: Eloralintide + Macupatide)
This therapeutic approach represents a strategic evolution in the obesity landscape by testing a “GLP-1-sparing” combination of eloralintide, a selective amylin receptor agonist, and macupatide, a selective GIP receptor agonist. Eloralintide mimics the hormone amylin (normally co-secreted with insulin) to centrally signal satiety and physically slow gastric emptying, targeting the same pathway as the “Cagri” component of Novo Nordisk’s CagriSema. Macupatide complements this by isolating the GIP pathway—the key metabolic differentiator of Lilly’s own tirzepatide—to enhance insulin sensitivity and lipid metabolism without direct GLP-1 receptor activation. By decoupling these mechanisms from the standard GLP-1 backbone, this fixed-dose combination aims to determine if targeting neuroendocrine satiety (Amylin) and metabolic efficiency (GIP) together can drive profound weight loss with a potentially differentiated tolerability profile.
This newsletter compiles publicly available information from company press releases and ClinicalTrials.gov. Not investment advice.